Ending HIV Transmission in Scotland by 2030: HIV Transmission Elimination Delivery Plan 2023-26
A detailed delivery plan outlining prioritised actions for the elimination of HIV Transmission in Scotland by 2030.
Tertiary Prevention - Treatment
Tertiary prevention relates to the linkage to and retention in care of people diagnosed with HIV, in order to provide accessible treatment including effective and easily tolerated antiretroviral therapy, which then prevents transmission referred to as “U=U” (Undetectable = Untransmittable). Uptake of treatment in those under care in Scotland is excellent and starting antiretroviral drugs soon after diagnosis - termed ‘treatment as prevention’ (TasP) - and partner notification is a routine part of care which, in turn, will reduce onward transmission of HIV. Work is required to establish the number of people who are living with HIV in Scotland but are not currently under care, and work to re-engage those who are not attending for care; termed ‘Trying to Find’ (TtF).
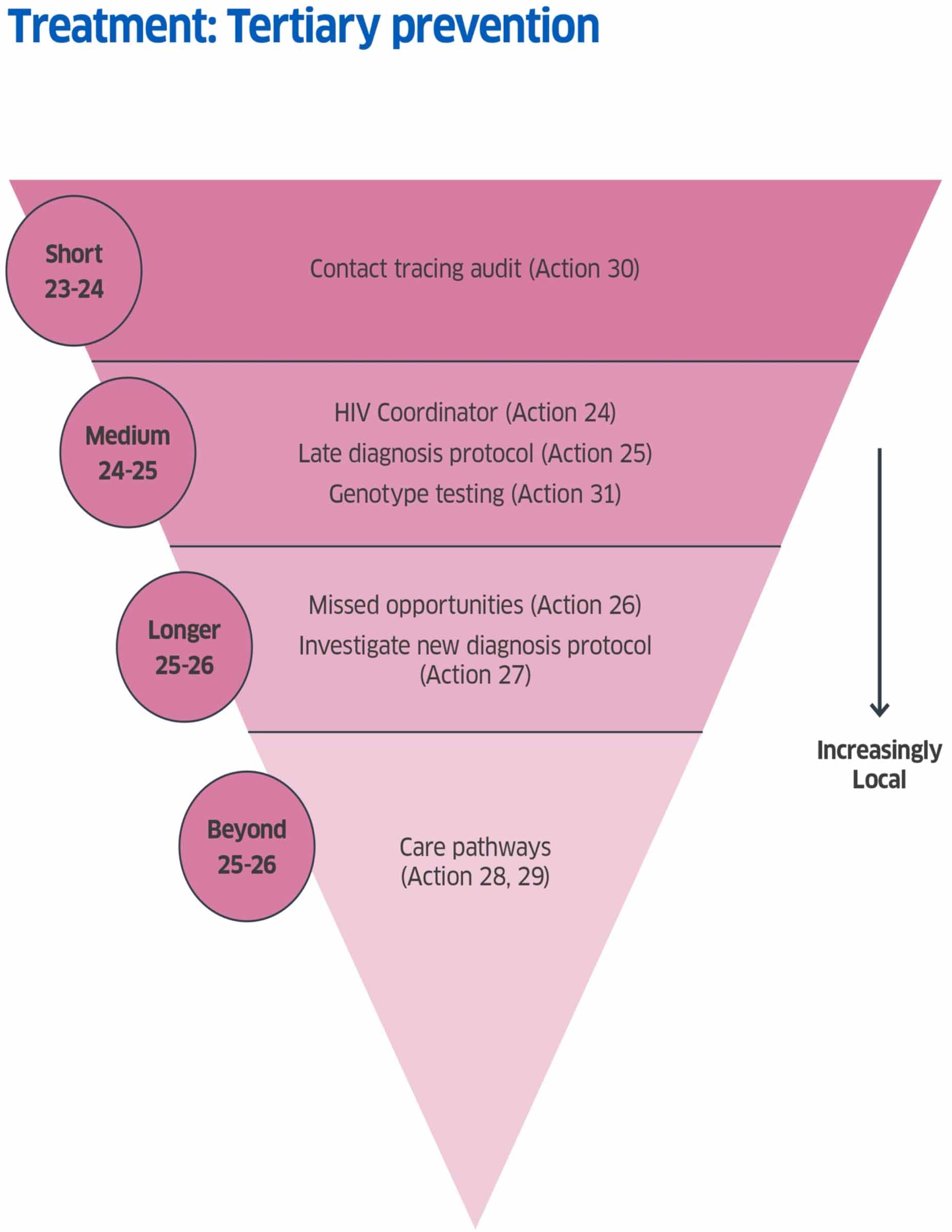
Tertiary Prevention Actions
HiTEOG 3.4 : Enhanced sexual health specialist service capacity to support clinical governance for HIV transmission elimination
Action 24: A funded National Coordinator post to oversee coordination of this Delivery Plan and to work with locally identified HIVTE Champions at NHS Board level towards delivery of actions to support elimination.
Delivery by: Scottish Government / PHS
Timeline: Medium term Commence: April 2024 Complete: March 2026
Detail: The coordination and delivery of a number of key actions relating to HIV transmission elimination across Scotland will fall to local clinical teams within HIV and Sexual and Reproductive Health services and public health colleagues. Extensive liaison with teams in acute medicine, primary care, emergency departments and support services such as laboratories will be required, as well as with other organisations and groups delivering actions against the various recommendations. A wealth of expertise in all these areas exists within the existing specialist clinical workforce, supported by BBV coordinators, Managed Clinical Network leads and other posts within some but not all NHS Boards, and the capacity to deliver when clinical pressures are substantial is constrained. The identification of local champions who can work together to drive progress and who are supported by a National Coordinator will provide the vital link across services and with centrally driven interventions.
HiTEOG 4.1: Episodes of late HIV diagnosis or diagnosis of HIV after death to be investigated using existing local clinical risk governance pathways for local learning reviews or significant adverse events, with supportive feedback and training offered if required.
Action 25: Take forward a nationally approved HIV Late Diagnosis protocol.
Delivery by: SHPN HIV Clinical Leads and PHS; Public Health Teams and Clinical Leads within NHS Boards.
Timeline: Medium term Commence: 2024/2025 Complete: On going
Detail: Late HIV diagnosis (first presentation linked to underlying immune deficiency and/or a CD4 cell count below 350) or death related to HIV in someone who was not diagnosed until after their death are potentially preventable and in cases where harm has occurred constitute significant adverse events (SAE) requiring formal investigation.
A late diagnosis protocol for Scotland was developed by a Short-Life Working Group of SHPN HIV Clinical Leads in 2019 and provides clear guidance on the approach to investigation and shared learning in cases of late diagnosis. Planned national governance arrangements were interrupted by the COVID-19 pandemic. Implementation of the protocol has been taken forward in several NHS Boards with varying success. As the number of people newly diagnosed with HIV falls further, each will become a ‘sentinel case’ – i.e. a case requiring further investigation to identify opportunities for prevention. Consideration will be given to a similar protocol to allow the investigation of all new diagnoses of HIV while maintaining anonymity and data protection (see action 27).
Action 26: To develop a proposal to collate and report key findings from local reviews of late HIV diagnosis to understand missed opportunities for diagnosis, with the aim of improving HIV testing services.
Delivery by: PHS and Clinical Leads
Timeline: Longer term Commence: 2025/26
Detail: Key findings on late diagnosis will help lessons learnt across Scotland. BHIVA & UKHSA have developed an online data entry system for late diagnosis and deaths, allowing data entry by individual clinics.
Action 27: Consider a protocol for the investigation of all new diagnoses of HIV.
Delivery by: PHS and HIV-TEDI.
Timeline: Longer term Commence: 2025/26
Detail: As new diagnoses become increasingly rare events, we will need to move towards a position where every new diagnosis is investigated to determine origin, and partner notification is undertaken. This will in some cases be an intensive ‘outbreak’ style response and will help to reduce a very low number of transmissions to zero, and continue monitoring any newly diagnosed infections beyond the elimination goal.
HiTEOG 4.2: To document defined care local pathways to support rapid entry into specialist HIV care after a positive test or access to primary combination prevention (if increased transmission risk identified) after a negative HIV test result.
Action 28: Local care pathways to be documented.
Delivery by: HIV-TEDI
Timeline: Beyond 2025/26
Detail: Local care pathways for people newly diagnosed with HIV are currently considered by HIV-TEDI to be robust and there is no evidence of systemic failure in linking people to care following diagnoses. Local services should continue to update their pathways in line with national and international guidance. A national review of pathways will be considered once work to provide a revised and expanded range of testing and prevention options is underway.
HiTEOG 4.3: To provide feedback to HIV care and treatment services when individuals relocate and enter care elsewhere (notably in another UK nation).
Action 29: Improvements to current systems to be considered.
Delivery by: TBC
Timeline: Beyond 2025/26
Detail: Initial discussions with the review group suggested that there is no evidence that there are significant gaps in current locally managed systems. Further improvements to existing processes including centrally coordinated data sharing could be considered in order to reduce the number of individuals who are recorded as ‘not under care’, but this is not an immediate priority and will be reviewed beyond 2025/26.
HiTEOG 5.1: To monitor and evaluate comprehensive contact tracing with partner support for people with newly diagnosed HIV in Scotland.
Action 30: Undertake a national Scotland-wide audit of HIV contact tracing.
Delivery by: SHPN HIV Clinical Leads with support from: Specialist Trainees in Genitourinary Medicine/Infectious Diseases/Specialist Sexual Health Advisers supported SHPN SRH Clinical Leads and HIV-TEDI
Timeline: Short term Commence: January 2024 Complete: December 2024
Detail: Contact tracing is an established pillar of clinical practice within SRH and HIV services and is given high priority in cases of HIV. Partner notification is highly skilled and time-consuming work undertaken by specialist sexual health advisers or trained nursing staff and is given high priority in cases of HIV. However, partner notification for people newly diagnosed with HIV is recognised as being challenging.
BASHH Standards on HIV contact tracing[xxii] were published in 2015 and UK guidelines on HIV contact tracing are currently being updated. As the number of new diagnoses of HIV in Scotland continues to fall, the importance of actions undertaken in connection with each person who is newly diagnosed – including partner notification (and tracing of contacts exposed via other routes, for example in PWID), supply of PEP and PrEP and potentially the use of phylogenetic analysis of HIV (which provides a genetic “fingerprint”) to link chains of transmission – assumes greater importance.
HIV partner notification activity has been maintained despite service pressures during and since COVID-19, but any variation in practice or performance across Scotland is not fully understood. The last UK national audit of HIV contact tracing was undertaken in 2018 and a Scottish national audit of sexual healthcare in people living with HIV was published in 2012. Similarly, the potential for enhancement or improvement of contact tracing activities is not well understood.
Audit findings will be carefully considered in conjunction with the monitoring and evaluation plan (see action 32), analysis of existing data, new research and international expert advice to ascertain the appropriate level of resource and energy to be applied to population measures such as mass testing versus intensive focused attention on individual and linked cases of HIV transmission. Clinical practice in contact tracing and protocols and information recording for investigation of HIV transmission in people newly diagnosed with HIV will be refined for use in conjunction with laboratory data, recognising at all times the need to prioritise the needs and opinions of the person diagnosed with HIV and the sensitivities around stigma and personally identifiable information.
Action 31: Development of a proposal for genotypic testing and phylogenetic analysis to support HIV transmission elimination activities.
Delivery by: PHS
Timeline: Medium term Commence: April 2024 Complete: March 2025
Detail: HIV-TEDI and Scottish Government will work with PHS to develop a proposal for genotypic testing and phylogenetic analysis. This will become particularly important as numbers become small and we move to a more focused approach of case linkage.
Contact
Email: SHBBV@gov.scot
There is a problem
Thanks for your feedback