Scotland's higher-activity radioactive waste policy: supplementary information
Provides information on radioactivity, radioactive waste and the regulatory framework that governs its management in Scotland.
SECTION 1 GUIDE TO RADIATION, RADIOACTIVITY AND RADIOACTIVE WASTE
Introduction
The purpose of this section is to provide some general information on radioactivity and to explain some of the terms that are used in the Scottish Government's Higher Activity Radioactive Waste Policy Consultation Document and more generally in discussion about radioactivity and radioactive waste issues. It is intended for those who may not be familiar with many of the technical terms used in discussing radioactivity and radioactive waste to help explain what we are talking about.
It is not intended to be a fully comprehensive, technical explanation of radioactivity and radioactive waste as there have already been many books written on this subject that can be referred to.
1.01 What is Radiation?
1.01.01 Radiation is the term used to describe energy which is transmitted, or radiated, in the form of particles, waves or rays which can travel through space. Radiation occurs all around us and examples of it include the heat given off by a household electric heater (thermal radiation) to stronger and potentially more harmful forms of radiation which can damage human cells. There are two categories of radiation: ionising radiation and non-ionising radiation.
What is ionising radiation?
Ionising radiation includes cosmic rays, x-rays, alpha particles, beta particles and gamma rays. Non-ionising radiation includes ultraviolet light, radiant heat, radiowaves and microwaves. When ionising radiation passes through matter it causes ionisation. This is the process by which a neutral atom within the matter becomes positively or negatively charged. This has the potential to damage cells and DNA, an important genetic material found in cells. Non-ionising radiation, however, does not produce ionisation when passing through matter and does not have enough energy to damage DNA directly.
This document refers to ionising radiation.
1.02 What is radioactivity?
1.02.01 Everything in the world is made up of extremely small building blocks called atoms. There are stable and unstable atoms. Unstable atoms change spontaneously to become more stable and during this process energy is released in the form of either particles (alpha and beta) or electromagnetic energy (gamma rays). This process of releasing energy is known as radioactivity while the energy itself is known as ionising radiation.
Figure 1: Illustration of an atom (courtesy NDA)
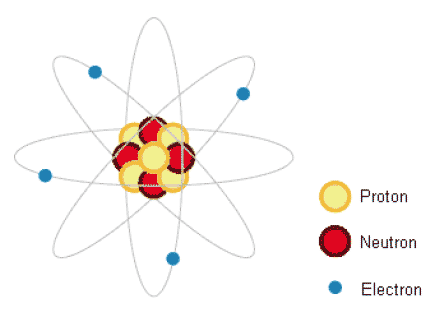
1.02.02 We are exposed to ionising radiation all of the time from natural sources. This is known as background radiation which includes radiation from rocks containing radioactive elements such as uranium, and cosmic rays from the Sun which enter the Earth's atmosphere. These levels can be higher in some places than in others. Background radiation can also come from naturally occurring radioactive elements that are present in our food and drink.
1.03 How do we use radioactivity?
Nuclear Power
1.03.01 Radioactive materials are used to produce electricity in nuclear power stations through the process of fission. If enough of the right kind of uranium is assembled in a nuclear reactor, the fission reaction produces heat which is used to create steam, which in turn drives turbines that generate electricity.
What is fission?
Nuclear reactors use the process of fission to produce power. Fission is the splitting of a nucleus into two or more pieces which can produce further chain reactions. Certain radioactive substances, such as Uranium, are known to undergo spontaneous fission at a low rate. In nuclear reactors uranium-235 is bombarded by neutrons to induce fission, this process produces energy, which is quickly converted to thermal energy, which is used to create steam, and in turn electricity.
Medicine
1.03.02 Ionising Radiation can be used in the diagnosis and treatment of diseases, but the benefit to the patient must outweigh the risk of exposure. Radiation such as x-rays can assist in the diagnosis of diseases by producing images of the inside of the body. The ability of ionising radiation to damage tissue can also be used to treat diseases such as cancers, where cancerous cells can be killed by a direct beam of ionising radiation.
Industry
1.03.03 Ionising Radiation is also used widely in industry for non-destructive testing, allowing various structures to be inspected for flaws and weaknesses without affecting their integrity. Because radioactivity can be easily detected radioactive tracers can be used to investigate areas which would otherwise be inaccessible, e.g. leaks in pipes, fractures in rocks. It can also be used in gauging applications, allowing the thickness or density of a product to be measured without damaging the product itself.
1.04 What are the health effects of radioactivity?
1.04.01 Ionising radiation can damage the tissues in our bodies through the process of ionisation. It can cause burns, cancer and hereditary effects and at very high doses radiation sickness. Because of this its use has to be carefully controlled. When people first began to work with radioactivity these effects were not known, however nowadays we can measure the dose a person receives, use shielding to control the source of the radiation, and protective clothing to ensure the safety of those who are at risk of exposure.
1.05 How are we exposed to radiation?
1.05.01 The greatest average exposure to ionising radiation that the public receives is from natural radiation and the largest contributor to this dose is radon gas. The greatest average artificial ionising radiation dose that we receive is from medical use in treatment and diagnosis, although the amount of radiation received is dependant on whether a person has undergone certain medical procedures that use ionising radiation. The public also receives small exposures to ionising radiation from consumer products such as smoke detectors, authorised discharges into the environment from hospitals, industrial premises and nuclear power stations, and historic fallout from nuclear weapons testing and nuclear incidents like Chernobyl.
Figure 2: Main routes of human exposure to sources of natural and man-made radiation (Courtesy SEPA)
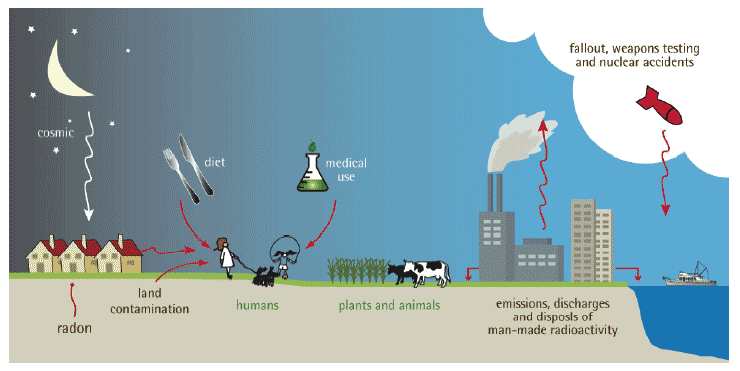
1.05.02 People receive a radiation dose from many sources, such as eating or breathing in naturally occurring radioactive materials, being exposed to external radiation naturally present in rocks and building materials and cosmic radiation from the sun.
Figure 3: Sources contributing to the average annual UK ionising radiation dose
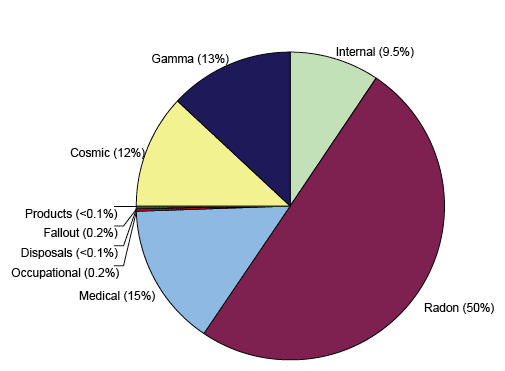
1.06 Types of Ionising Radiation
1.06.01 There are three main types of radiation:
- Alpha (a) radiation can be the most harmful if breathed in or eaten. However, it can only travel a few centimetres in air and can be stopped by a thin layer of material such as paper, clothing or the skin so is less harmful if it is outside the body.
- Beta (ß) radiation is less harmful than alpha if breathed in or eaten, but can travel further in air and is more penetrating through material. It passes through paper and needs a few millimetres of aluminium or a thin piece of lead to stop it. Similarly it can pass through the skin from outside the body to affect the internal organs.
- Gamma (?) radiation can travel a long way through air and is extremely penetrating: several centimetres of lead or metres of concrete are required to stop it. However, it gets weaker as you move away from the source. This means that it can easily pass through the skin into the body and affect internal organs.
1.06.02 Alpha and beta radiation take the form of particles that are emitted from radioactive material whereas gamma radiation is a form of electromagnetic radiation that is emitted as a wave of energy. This electromagnetic spectrum includes light, ultraviolet rays and X-rays.
1.06.03 We can be protected from external radiation by various levels of shielding .
Figure 4: Illustration of shielding from radioactivity (redrawn by Bell Design)
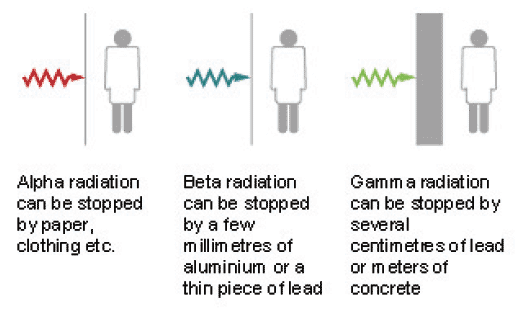
1.06.04 A nuclear reactor produces substances which give off all three types of radiation: its core is very radioactive. Workers are protected from this radiation by shielding the core with steel and concrete.
1.06.05 All radioactive materials (including the radioactive wastes produced by nuclear reactors, industry, medicine and defence agencies) require careful handling and monitoring to protect people and the environment.
1.07 Will radioactivity go away?
1.07.01 The amount of ionising radiation given off by a radioactive substance will gradually decrease over time. This is due to a process called radioactive decay. The time it takes for the radioactivity to decrease by 50% is called its ' half-life'. Different radioactive materials have different half-lives: some can be a matter of seconds, some many thousands of years but the amount of radioactivity will always reduce as time goes on.
1.07.02 Radioactive decay and a material's half life are of importance as it can be used to help determine what the best option for dealing with radioactive materials should be. Waste which is highly radioactive but has a short half life may not be dealt with in the same way as waste of a similar radioactive level which has a half life of many thousands of years.
Figure 5: Graph of a radioactivity decay curve (courtesy NDA)
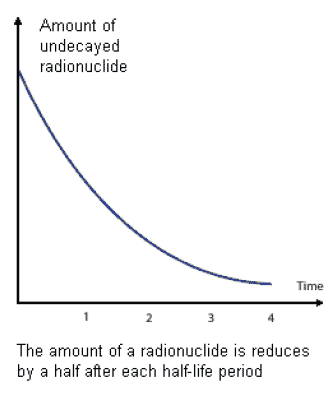
1.07.03 This means that radioactive waste of a certain activity can be stored for a period of time until it decays to below an activity level at which it could potentially be reclassified and disposed of at existing facilities.
1.07.04 Each time a radionuclide decays (i.e. emits a neutron, proton or electron) its chemical composition changes. This means each time it decays it becomes a different radionuclide. The original radionuclide is known as the parent and the resulting radionuclide(s) are called daughter products. This sequence of events can be repeated many times over, resulting in a radioactive decay chain.
What is a radionuclide?
A nuclide is a certain species of atom with a particular composition of protons, neutrons and electrons. A radionuclide is an unstable nuclide which emits ionising radiation. For example Thorium-234 and Radon-222 are different radionuclides, uranium-238 and uranium-234 are also different radionuclides as they have different compositions of protons and neutrons.
1.07.05 Radioactive decay chains are relevant to the storage and disposal of radioactive waste as the original material placed in a facility will decay to become a different daughter product which could be solid, liquid or gaseous forms. Some of these daughter products may themselves be radioactive but others will be non-radioactive elements.
1.08 How does Material become Radioactive?
1.08.01 Some material is naturally radioactive; however some materials can become radioactive either by being contaminated, activated or both.
1.08.02 Material can become contaminated or activated through medical, industrial and nuclear processes where it comes into contact with radiation.
Contaminated materials
1.08.03 Radioactive contamination is caused by radioactive material being deposited on the surface of objects. The radioactivity may be deposited from airborne sources, from waterborne sources, or from physical contact. Radioactive contamination is generally located on or near the surface of materials like metal or high-density concrete or painted walls. Radioactive contamination can usually be removed from surfaces by washing, scrubbing, spraying, or by removing the outer surface of the contaminated objects.
Activated materials
1.08.04 Activated products are radioactive materials that are created when stable substances are bombarded by neutrons that make them radioactive. Typically these are produced from elements, contained within the steel structure of nuclear reactors.
1.09 What is Radioactive Waste?
1.09.01 The various uses of radioactive substances results in the production of radioactive waste. Any material contaminated by or incorporating radioactivity above certain thresholds defined in legislation, and for which no further use is envisaged, is known as Radioactive Waste. In practice this often comprises of everyday items that have become contaminated by contact with radioactive materials, concrete and other building materials from decommissioning buildings on nuclear sites and components that have become activated in nuclear reactors.
1.09.02 This radioactive waste falls into four categories:
- High Level Waste ( HLW),
- Intermediate Level Waste ( ILW),
- Low Level Waste ( LLW), and
- Very Low Level Waste ( VLLW).
1.09.03 There are government policies and regulations to determine how these wastes are managed. For the purpose of these policies, radioactive waste is described as "higher activity waste" or "lower activity waste". Higher activity waste is high and intermediate level radioactive waste, and lower activity waste is low and very low level radioactive waste.
1.09.04 The following diagram shows the relationship between the different categories of radioactive waste and how they are classed as higher activity or lower activity waste.
Figure 6: Diagram of Waste Categories (Source Scottish Government)
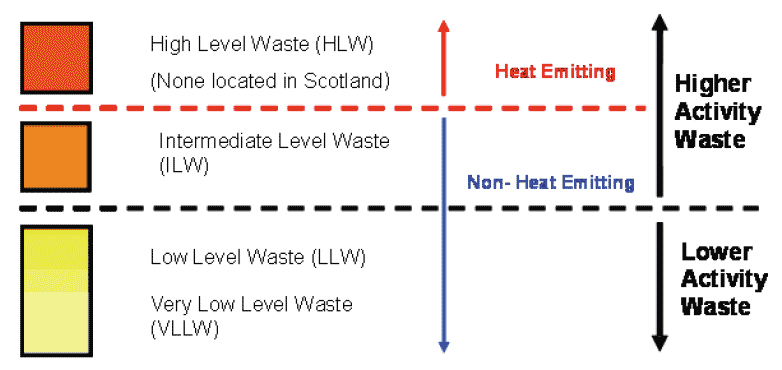
1.10 Radioactive Waste Categories
High Level Waste ( HLW)
1.10.01 Waste that contains sufficiently high levels of radioactivity that heat is generated and this needs to be considered during its storage and disposal.
Intermediate Level Waste ( ILW)
1.10.02 Waste containing higher concentrations of radioactivity than low level waste, but without the heat generation that occurs in high level waste. These wastes are typically from routine power station maintenance and decommissioning operations and typically consist of metal items such as nuclear fuel casing and nuclear reactor components, graphite from reactor cores, and sludges from the treatment of radioactive liquid effluents.
Low Level Waste ( LLW)
1.10.03 Wastes that are within specified concentrations of radioactivity: lower than the levels set for intermediate level waste but higher than the levels set for very low level waste. These wastes may arise from the non-nuclear and nuclear industries and typically consist of everyday items that have become contaminated during use by contact with radioactive materials, and waste from decommissioning operations such as building rubble, soil and metal items such as framework, pipework and reinforcement
Very Low Level Waste ( VLLW)
1.10.04 A sub-category of LLW is Very Low Level Waste ( VLLW). This is radioactive waste that mainly arises from non-nuclear industries such as hospitals, universities and industrial premises and can be safely disposed of with ordinary (non-radioactive) waste. Typically this waste consists of everyday items used in the handling of radioactive materials such as gloves, vials, paper towels and other laboratory and medical equipment.
There is a problem
Thanks for your feedback